Newsletter
VOCs are important indoor air pollutants that negatively impact our health. We've talked about what exactly VOCs are in our previous post, and now we are facing another key question: how are VOCs measured, or how does a TVOC sensor work?
If you're looking to monitor indoor air quality in a building you manage, you should check out Kaiterra's air quality monitors - used at Google, Microsoft, Facebook, and leading real-estate companies. Grab some time with a Kaiterra air quality expert to discuss your project and share best practices for measuring and improving IAQ: 
There are a number of ways to measure VOCs, with pros and cons based on the situation, budget available, etc. Historically, laboratory techniques such as flame ionization detectors or gas chromatography–mass spectrometry were used, providing an accurate way to identify specific gases within a sample.
While lab-based measurements may be highly accurate, they are unable to provide a continuous measurement of TVOC, which is incredibly important and, some may even argue, more important than having a perfectly accurate value for a specific gas.
What Is an MOS Sensor?
For the continuous monitoring of TVOC, MOS sensors are generally used. This is a broad category, and sensors across this category provide varying degrees of quality in their readings - not all MOS sensors are built equal. Similar technology is used throughout, however, which is what we will explore below:
MOS (metal oxide semi-conductors) work by heating a thin film, or surface, of metal-oxide particles. In Kaiterra products, this is a thin film of metal-oxide nanoparticles. This film is heated to around 300’C (hence the warm up period after turning on a Kaiterra device).
During operation, oxygen particles will be adsorbed on the surface, and these in turn will react with the target gas. This reaction causes a release of electrons from the oxygen present on the surface, which in turn leads to a change in electrical resistance of the metal-oxide layer.

Chemical reactions within the MOS sensor creates a change in electrical resistance.
What the sensor is actually measuring is the electrical resistance of this metal-oxide layer. This real time measurement and output of resistance is the first step in obtaining our TVOC reading.
The change in resistance across the surface of the sensor is directly proportional to the change in concentration of the target gas(es). The exact degree to which the resistance will change (that is, the ratio or equation that explains this relationship) may vary with the exact gas present in the space. For example, formaldehyde and ethanol will have a similar relationship, but it may not be identical.
The sensor will be sensitive to a wide variety of VOCs, rather than to one specific individual VOC.
How does the change in resistance become a TVOC reading in ppb?
Although similar, different VOCs will have slightly different reactions - the same increase in formaldehyde and in ethanol might not lead to the exact same change in resistance on the sensor. This means that for a change in resistance to be mapped to a change in VOC levels in ppb, either the exact gas in the air must be known, or an assumption about the composition of the air in the space must be made.
Mølhave et al. defines a “Typical IAQ Mix” of 22 VOCs at concentrations similar to those determined on average in residential indoor environments. This Typical IAQ Mix is used to interpret the change in resistance on the sensor’s film, and convert it into a TVOC reading in ppb.
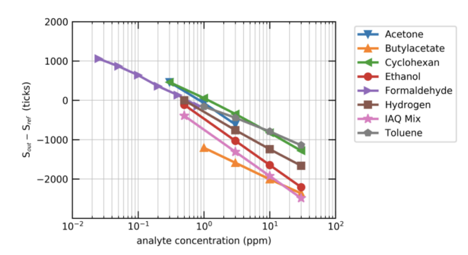
Image via https://www.mendeley.com/catalogue/2e5a9220-a6a4-3330-8dd8-7933e8fd52a6/
This methodology allows for continuous monitoring, a long lifespan of the sensor, and readings in ppb that, when used in a typical indoor space, very closely match measurements taken with laboratory methodologies.
Learn more about TVOC and how it fits into indoor air quality by downloading our free eBook, Indoor Air Quality 101 below:






.png?width=200&height=148&name=Menu%20C%20(2).png)

.png?width=307&height=228&name=Menu%20-%20D%20(1).png)
.png)





